Elements Atoms and Subatomic Particles, Oh My!
Welcome to my disciplinary text set used to teach my 11th grade chemistry class. This unit focuses on teaching about elements, atoms, and subatomic particles. The print texts I have selected are quite dense and complex. Because of this, I have a definite order of events within this text set. I have listed them in the order they will appear while teaching this unit and detailed in my summaries how this will help to set up my students to tackle the print readings.
Text #1- Culturally Relavant- Video
Text #2- Multimedia- Infographic
"Elements Infographic," Schreiber, Amanda, 2017.
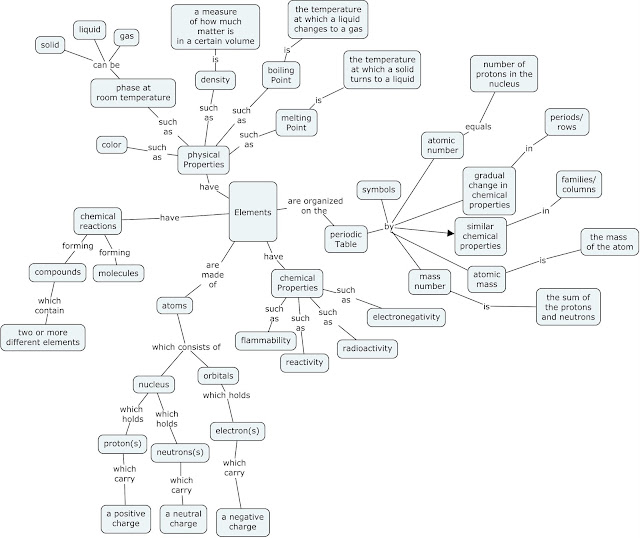
Summary- My infographic is a preview or a teaser, if you will, as to what is going to come throughout this lesson. It begins with a very basic description of elements making up all matter. From there it goes into a comic with Greek philosophers and historical scientists arguing their atomic theories. Next is a simple section on the three subatomic particles within each atom and finally a look at what the periodic table looks like and some information on how each element is organized on the table. I want the students to have a visual of the concepts prior to really diving into them to help activate their thinking as we move forward.
My text set consists of 3 multimedia, 3 print, and 2 culturally relavent texts which I would use to teach the beginnings of elements, atoms, and subatomic particles. The limit of texts we could use was 8, however I found two more I would like to use with this unit. Stay tuned for my next blog post to see which two multimedia texts did not make the cut!
Text #1- Culturally Relavant- Video
"Meet the Elements" They Might Be Giants, October 6, 2009 Online Video https://www.youtube.com/watch?v=Uy0m7jnyv6U
Summary- This video is a animation set to a song. It introduces the concept of elements and how everything is made of them. It also shows that elements join together to form other things. It does this in a very student friendly way by bringing in topics they can relate to based on their background knowledge. It has a very catchy tune and the chorus is repeated several times which will help the students remember some of the vocabulary words that are introduced. I believe that this video (text) is culturally relevant as it relates to an adolescent culture. The video is housed on youtube.com which is a website widely used by adolescents. This connects them from their home life and outside interests to the text. It also uses music and animation to engage the students in learning.
Quantitative text analysis- I typed the lyrics of the song into StoryToolz which gave it an average grade level of 20.
Qualitative text analysis- I find the quantitative analysis to be very misleading. I rate this video at an 8th grade level.
In most of the areas of the qualitative rubric I would rate this as slightly complex using the following criteria:
- The text is structured with simple graphics and in a sequential order. There are connections between all of the ideas making it very easy for the viewer to follow.
- The purpose is very clear that the video is an introduction to the elements.
- The knowledge demands are simple. While the lyrics themselves contain some advanced vocabulary, the ability of the students to relate to it relies on common knowledge.
- I would rate the language features moderately complex only because of the vocabulary. As a introductory text, the names of the elements will be fairly unknown to students which makes that part of the language very complex. However the conventionality and sentence structure are only slightly complex.
Task Complexity- My class that consists of 11th graders will have heard many of the element names introduced in the video in prior classes. The fun video is to really re-introduce the topic and to motivate interest. Hearing the names of the elements and how they relate to everyday objects in the students lives will reactivate what they have learned during their middle school science classes.
Text #2- Multimedia- Infographic
"Elements Infographic," Schreiber, Amanda, 2017.
Summary- My infographic is a preview or a teaser, if you will, as to what is going to come throughout this lesson. It begins with a very basic description of elements making up all matter. From there it goes into a comic with Greek philosophers and historical scientists arguing their atomic theories. Next is a simple section on the three subatomic particles within each atom and finally a look at what the periodic table looks like and some information on how each element is organized on the table. I want the students to have a visual of the concepts prior to really diving into them to help activate their thinking as we move forward.
Text # 3 Multimedia- Video
.
"What is an Atom?" Emerald Robinson, November 13, 2102, https://www.youtube.com/watch?v=o-3I1JGW-Ck
Summary- This is a video that explains and shows that elements are made of atoms and then goes further to explain that atoms are made of sub particles. This will help the students visually see where these particles are within the atom, define their names and charges. The animation shows the electrons movement around the nucleus which is difficult to explain or read. The visual helps to understand what that looks like. The video then goes into explaining atomic number and atomic mass which is a great preview for learning the periodic table and what information we will find there.
Summary- This is a video that explains and shows that elements are made of atoms and then goes further to explain that atoms are made of sub particles. This will help the students visually see where these particles are within the atom, define their names and charges. The animation shows the electrons movement around the nucleus which is difficult to explain or read. The visual helps to understand what that looks like. The video then goes into explaining atomic number and atomic mass which is a great preview for learning the periodic table and what information we will find there.
Quantitative Text Analysis- Storytoolz rated this video at an average grade level of 10.2.
Qualitative Text Analysis- 10-11th grade level
- The text structure of the video is moderately complex. The video is very organized and follows a sequential order. The text features and graphics really enhance the viewer's understanding of the content.
- The language features are very complex. I rated it as such based on the subject-specific vocabulary.
- The purpose and knowledge demands are very complex based on the abstract purpose of the text. Atoms are something that none of the students can physically see so having to imagine it makes it difficult. The visuals in the video make it more accessible for them to grasp. There is a large amount of discipline-specific knowledge.
Task Complexity- The purpose of this video is to solidify the fact that elements are made of atoms. This video also provides a visual representation of the sub particles along with introducing some information about what is noted on the periodic table. I feel this is a great bridge from the preview of subatomic particles they received viewing the infographic to a lesson on the periodic table.
Text #4- Print
"The Elements," University of South Carolina- Upstate, https://chem.libretexts.org
Summary- This article gives an overview of what an element is and then talks about the abundance of specific elements in the universe and more specifically on earth. Then, the article talks about the elemental composition of the human body. What better way to let it sink in that elements make up all matter than to relate to my students bodies? This takes a very abstract concept- that elements are what makes up everything in their world, to a really concrete example. Without the elements, even they wouldn't exist. The article also provides a table with the names of the elements and their corresponding symbols.
Summary- This article gives an overview of what an element is and then talks about the abundance of specific elements in the universe and more specifically on earth. Then, the article talks about the elemental composition of the human body. What better way to let it sink in that elements make up all matter than to relate to my students bodies? This takes a very abstract concept- that elements are what makes up everything in their world, to a really concrete example. Without the elements, even they wouldn't exist. The article also provides a table with the names of the elements and their corresponding symbols.
Quantitative Text Analysis- Storytoolz rated this text at an average grade level of 11.8.
Qualitative Text Analysis- 11-12th grade level
- The text structure is moderately complex. The organization is sequential and the graphics are supplementary to the text, but not integral to understanding the text.
- The conventionality, vocabulary and sentence structure of the language features is very complex. The vocabulary is unfamiliar and subject specific. For example, the section on abundance assumes the reader knows and understands that most elements are naturally occurring on earth and that they make up specific parts of the earth.
- The purpose of the text is subtle and very complex. There would have to be some front-loading prior to reading this text and a reading activity while reading the text so the students know what information I want them to take from it.
- The knowledge demands are very complex. There is prior knowledge required that includes knowing specific parts of the earth and reading tables containing scientific data.
Task Complexity- My purpose for choosing and using this text would be to teach my students the abundance of elements on earth and in the body. This reading would come after learning the basics of elements and atomic structure. This text would be coupled with an activity where students calculate the percentage of the elements in THEIR body based on their specific weight.
Text #5 - Print
https://chem.libretexts.org/LibreTexts/University_of_South_Carolina_-_Upstate/USC_Upstate%3A_Chemistry_of_Life_(Mueller)/02%3A_Elements%2C_Atoms%2C_and_the_Periodic_Table/2.2%3A_Atomic_Theory
"Atomic Theory," University of South Carolina- Upstate, https://chem.libretexts.org
Summary- This print text describes John Dalton's atomic theory and goes into a small part of what Greek philosophers originally introduced as as atomic concepts. It also discusses how small atoms are. This piece will form a connection to the infographic I have the students look at as their preview because it names some of the names from the "comic" portion where the philosophers and scientists are speaking about their theories.
Text #6 - Print
https://chem.libretexts.org/LibreTexts/University_of_South_Carolina_-_Upstate/USC_Upstate%3A_Chemistry_of_Life_(Mueller)/02%3A_Elements%2C_Atoms%2C_and_the_Periodic_Table/2.3%3A_The_Structure_of_Atoms
"The Structure of Atoms," University of South Carolina- Upstate, https://chem.libretexts.org
Summary- This text finalizes the history and structure of the atom by describing Rutherford's discovery of the nucleus and how electrons orbit it. Once the students read this, the atomic structure and sub particles of the atom will be understood.
Text # 7 - Multimedia - Visual Aide/Slide
Text #5 - Print
https://chem.libretexts.org/LibreTexts/University_of_South_Carolina_-_Upstate/USC_Upstate%3A_Chemistry_of_Life_(Mueller)/02%3A_Elements%2C_Atoms%2C_and_the_Periodic_Table/2.2%3A_Atomic_Theory
"Atomic Theory," University of South Carolina- Upstate, https://chem.libretexts.org
Summary- This print text describes John Dalton's atomic theory and goes into a small part of what Greek philosophers originally introduced as as atomic concepts. It also discusses how small atoms are. This piece will form a connection to the infographic I have the students look at as their preview because it names some of the names from the "comic" portion where the philosophers and scientists are speaking about their theories.
Text #6 - Print
https://chem.libretexts.org/LibreTexts/University_of_South_Carolina_-_Upstate/USC_Upstate%3A_Chemistry_of_Life_(Mueller)/02%3A_Elements%2C_Atoms%2C_and_the_Periodic_Table/2.3%3A_The_Structure_of_Atoms
"The Structure of Atoms," University of South Carolina- Upstate, https://chem.libretexts.org
Summary- This text finalizes the history and structure of the atom by describing Rutherford's discovery of the nucleus and how electrons orbit it. Once the students read this, the atomic structure and sub particles of the atom will be understood.
Text # 7 - Multimedia - Visual Aide/Slide
http://slideplayer.com/slide/8021051/
Summary- This is a visual aide I will present to the class to teach them what the numbers and symbols on the periodic table stand for and how to calculate the number of neutrons. This will be followed by an activity with filling in some element boxes on their own.
Text #8- Culturally relevant - Chemistry Crack App
"Chemistry Crack", Day, Jennica; March 4, 2105.
Summary- This app is an interactive game that asks students multiple choice questions. I find this culturally relevant as it related to adolescent culture. Adolescents use iPads, tablets, and phones for many reasons- two of the biggest reasons is for communication and playing games. This app will be used as a fun break from the heavy print readings we have read and will help to continue to build the structures necessary for meaningful learning of these concepts.
The app is split into different categories:
The app is split into different categories:
For our lesson on elements we would use the atomic structure category. The game lists a question and students choose their answer. It gives automatic feedback as to whether you have answered correctly.
My text set consists of 3 multimedia, 3 print, and 2 culturally relavent texts which I would use to teach the beginnings of elements, atoms, and subatomic particles. The limit of texts we could use was 8, however I found two more I would like to use with this unit. Stay tuned for my next blog post to see which two multimedia texts did not make the cut!











Hey Mandy, I love the texts you chose! you have such a nice variety of written, video, and interactive/multimedia texts, I am definitely going to be using some of these in my classroom :D
ReplyDeleteThanks! If you are a 2048 fan, check out the periodic 2048 app. Not so good for teaching chemistry, but great for wasting time!
DeleteI can tell you have given a lot of thought into when you are going to introduce each text. I love the videos and that app that you have! I am definitely going to keep these in mind for my classroom!
ReplyDeleteThis is a really nice blog post, Mandy. I can tell you put a lot of effort into this. I enjoyed how you actively worked to demonstrate how these texts would relate to each other in your own classroom. That shows you put a lot of planning into this. It is pretty clear that any student wandering onto here is going to learn lots!
ReplyDeleteOoh! And I know you already finished your scaffolding project, but I found this on Reddit and thought of you:
ReplyDeletehttp://vis.sciencemag.org/chemhaiku/?utm_source=sciencemagazine&utm_medium=twitter&utm_campaign=6350issue-14513
hover over the element and it gives you a haiku based on the element! (Magnesium is my favorite, seems extra poetic lol) just thought it might make a good addition to your personal list of atom-related stuff :D